Abstract
Background
Natural Killer (NK) lymphocytes possess innate anti-tumor activity that has the potential to be used as an allogeneic cell therapy due to reduced GvHD risk relative to αβ T cells. Despite their potential, adoptive NK cell immunotherapies have been limited by poor expansion in vivo. Using our previously developed Chimeric Antigen Receptor-T cell (CAR-T) strategy that relies on rimiducid-based dimerization of inducible MyD88/CD40 (iMC) to regulate T cell expansion and survival, we demonstrate that iMC can also be applied to NK cell growth and anti-tumor efficacy in vitro and in vivo. Moreover, a rapamycin-inducible Caspase-9 (iRC9) was used to provide an orthogonally regulated safety switch.
Methods and Results
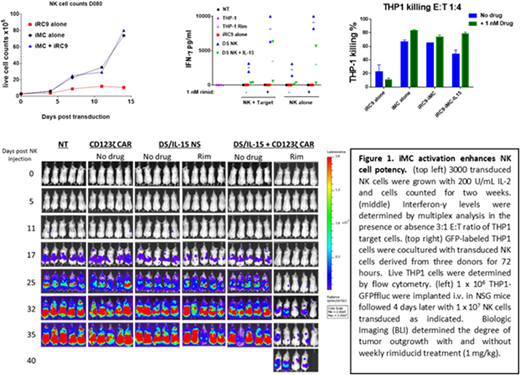
CD56+ NK cells were isolated from peripheral blood of human donors, stimulated overnight with IL-15 then activated by seeding with K562 erythroleukemia target cells. NK cells were then transduced with γ-retrovirus encoding control iRC9-2A-ΔCD19, iRC9-2A-ΔCD19-2A-iMC (dual-switch NK) or iRC9-2A-IL-15-2A-ΔCD19-2A-iMC (dual-switch/IL-15 NK). ΔCD19 marked transduced cells in 50:50 cocultures with untransduced NK cells. NK cells containing only iRC9 grew at the same rate as untransduced cells, but iMC-expressing NK cells displayed enhanced growth that was further augmented by 1 nM rimiducid treatment. In cocultures with THP1 acute myeloid leukemia cells at increasing Target:Effector (T:E) ratios, presence (P < 0.001, two way ANOVA) and activation (P <0.001) of iMC increased tumor killing activity. Inflammatory cytokine and chemokine production was also dramatically (10 to 1000-fold) elevated by the expression and activation of iMC in NK cells in the presence and absence of THP1 tumor target.
To study in vivo anti-tumor activity, immunodeficient NSG mice were engrafted with dual-switch NK cells with or without autocrine IL-15 expression in the presence or absence of THP-1 tumor targets. When tumor was present, unstimulated iMC with IL-15 or activation of iMC without IL-15 expression supported modest NK cell expansion, but rimiducid stimulation of iMC plus autocrine IL-15 showed enhanced NK expansion in vivo. Furthermore, in tumor-free animals only dual-switch/IL-15 NK cells with weekly rimiducid stimulation expanded and persisted in vivo (up to 7 weeks). Cotransduction of a first generation CD123-targeted CAR to produce dual-switch/IL-15 CD123CAR-NK cells led to rimiducid-dependent control of THP1 tumor outgrowth in vivo beyond 40 days. Conversely, temsirolimus-mediated activation of the iRC9 safety switch rapidly (< 24 hours) ablated dual-switch NK cells in vivo.
Conclusions
Inducible MyD88/CD40 is an activation switch that supports NK cell expansion, persistence and anti-tumor activity. When paired with autocrine IL-15 expression, this platform supports NK expansion and persistence in vivo, and AML tumoricidal activity that can be further activated by target-specific CAR expression. Moreover, the fast-acting, orthogonally regulated proapoptotic switch, iRC9, mitigates the risk of off-tumor targeting. Therefore, we describe a novel, regulated NK cell platform that solves many of the challenges of NK cell-based therapy and should be amenable to a readily translatable off-the-shelf cellular therapy for malignancies.
Wang:Bellicum Pharmaceuticals: Employment, Equity Ownership. Chang:Bellicum Pharmaceuticals: Employment, Equity Ownership. Jasinski:Bellicum Pharmaceuticals: Employment, Equity Ownership. Medina:Bellicum Pharmaceuticals: Employment, Equity Ownership. Zhang:Bellicum Pharmaceuticals: Employment, Equity Ownership. Foster:Bellicum: Employment, Equity Ownership. Spencer:Bellicum Pharmaceuticals: Employment, Equity Ownership. Bayle:Bellicum Pharmaceuticals: Employment, Equity Ownership.
Author notes
Asterisk with author names denotes non-ASH members.
This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal